Fortus® 450mc

Revolutionize medical device production and prototyping with the Stratasys® Fortus® 450mc, offering high-precision 3D printing in both biocompatible and non-biocompatible materials.
-
FDM® (Fused Deposition Modelling) Printing Technology
-
406 x 355 x 406 mm Build Volume
-
127 - 330 µm Layer Thickness
-
Unlimited (using OpenAM™) with 19+ validated materials Material Compatibility
-
Auto changeover - 2 bays each (material & support) Material Delivery
Precise and Versatile
With layer thicknesses as fine as 0.127 mm, the Fortus® 450mc offers remarkable accuracy, which is essential for producing intricate medical models and equipment. Producing anatomically correct models for surgery planning, specialized implants, and even complex dental accessories requires this degree of accuracy.
It works with a variety of biocompatible materials, such as ABS-M30i, PC-ISO, and ULTEM™ 1010, which are perfect for creating prototypes and medical equipment that need to be sterilized. Because of its strong heat and chemical resistance, ULTEM™ 1010 in particular is appropriate for autoclave sterilization.
This adaptability enables healthcare providers to precisely produce surgical guides, patient-specific anatomical models, and customized medical instruments. To increase surgical accuracy and possibly improve patient outcomes, orthopedic doctors, for example, can utilize the printer to produce personalized surgical guidelines for joint replacement surgeries.
Large Build Format
The Fortus® 450mc's large 406 x 355 x 406 mm build chamber makes it possible to produce larger medical equipment and full-scale anatomical models. This capability improves efficiency in medical procedures and dentistry, especially when it comes to making several instruments or detailed surgical planning models in a single build.
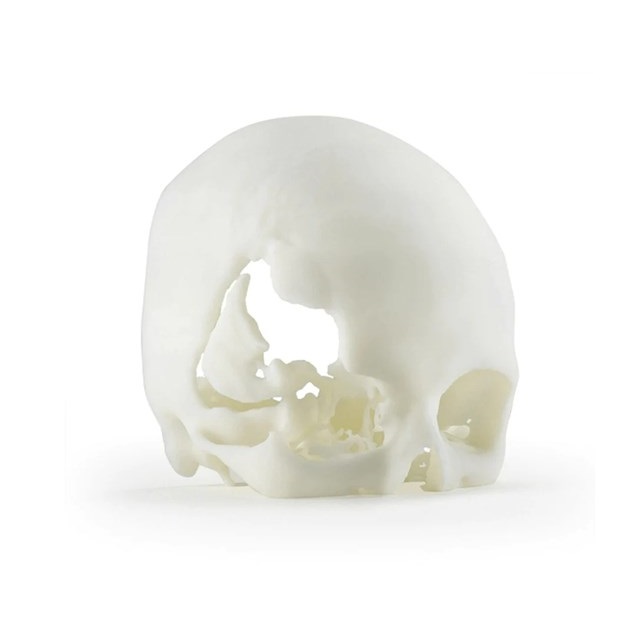
For instance, a maxillofacial surgeon could print a full-size skull model for intricate reconstructive surgery planning or a dental lab may create several sets of dental models in a single print job, greatly cutting down on production time and expenses.
Additionally, because of the huge build volume, functioning prototypes of larger medical devices, such custom orthoses or prosthetic limbs, can be created, allowing for more thorough testing and evaluation prior to final manufacturing.
Meets Safety and Quality Standards
The Fortus® 450mc is suitable for medical applications because it was created with safety and regulatory compliance in mind. It ensures that printed parts meet the strict requirements for medical use by supporting materials that adhere to ISO 10993-5/-10 standards for biocompatibility. For medical device manufacturers hoping to prototype and manufacture end-use components that comply with industry standards, this functionality is essential.
FDA standards for medical device production are also supported by the printer's ability to maintain consistent part quality and traceability. This compliance helps academic medical institutes that conduct clinical research by guaranteeing that 3D-printed models or equipment used in studies fulfill the required safety and quality requirements, which makes it easier to translate research into clinical practice.
Advanced Software and Workflow Integration
The workflow from design to production is streamlined by the Fortus® 450mc's compatibility with GrabCAD Print software and other specialized solutions. This makes it easier for medical practitioners to handle intricate anatomical data from CT or MRI scans, which enables them to create patient-specific models and devices more quickly.
The traceability requirements that are frequently necessary in the manufacturing of medical devices are also supported by the integration capabilities. To ensure quality control and regulatory compliance, the biomedical engineering department of a hospital, for example, may easily enter patient scan data, design a custom implant, and print it with complete documentation of the process.
Medical practitioners can devote more time to patient care and less time to technical matters by using the capability of the program to automatically optimize part orientation and support structures.
Can Use Engineering-Grade Thermoplastics
The Fortus® 450mc is a great tool for creating robust, functional prototypes and end-use parts for medical applications because it can print using engineering-grade thermoplastics.
Strong medical devices that can survive rigorous testing and practical usage can be made with materials like ULTEM™ 9085, which have excellent strength-to-weight ratios and flame, smoke, and toxicity (FST) ratings. Medical device firms find this feature especially useful throughout the testing and prototyping stages of product development.
A company creating a novel surgical tool, for instance, may print working prototypes that closely resemble the finished product's mechanical characteristics. This would enable thorough testing of the product's durability, functioning, and ergonomics prior to mass production. For academic medical centers, researchers can use this feature to create custom research tools or experimental devices that can withstand repeated use and sterilization, enhancing the quality and reliability of their studies.
Applications
Anatomical Models
Manufacturing patient-specific anatomical models using the printer's high precision and biocompatible materials for surgical planning and education.
Tools and Guides
Creation of patient-specific surgical guides for procedures like joint replacements or implants, enhancing surgical accuracy and potentially reducing recovery times.
Medical Device Prototypes
Rapidly iterate designs and produce functional prototypes for testing, accelerating the development process and reducing time-to-market for new devices.
Custom orthoses and prosthetics
Create patient-specific orthotic devices and prosthetic components that can be customized for optimal fit and function.
Compatible Materials


PC-ISO

Stratasys® ULTEM™ 1010

Stratasys® ULTEM™ 9085

FDM Nylon 12

Stratasys® FDM Nylon 12CF (Carbon Fiber)

Stratasys® PC-ABS

Stratasys® PC (Polycarbonate)

Stratasys® ABS-ESD7™

ABS-M30

Antero 800NA

Antero 840CN03

Stratasys® PC-ESD





