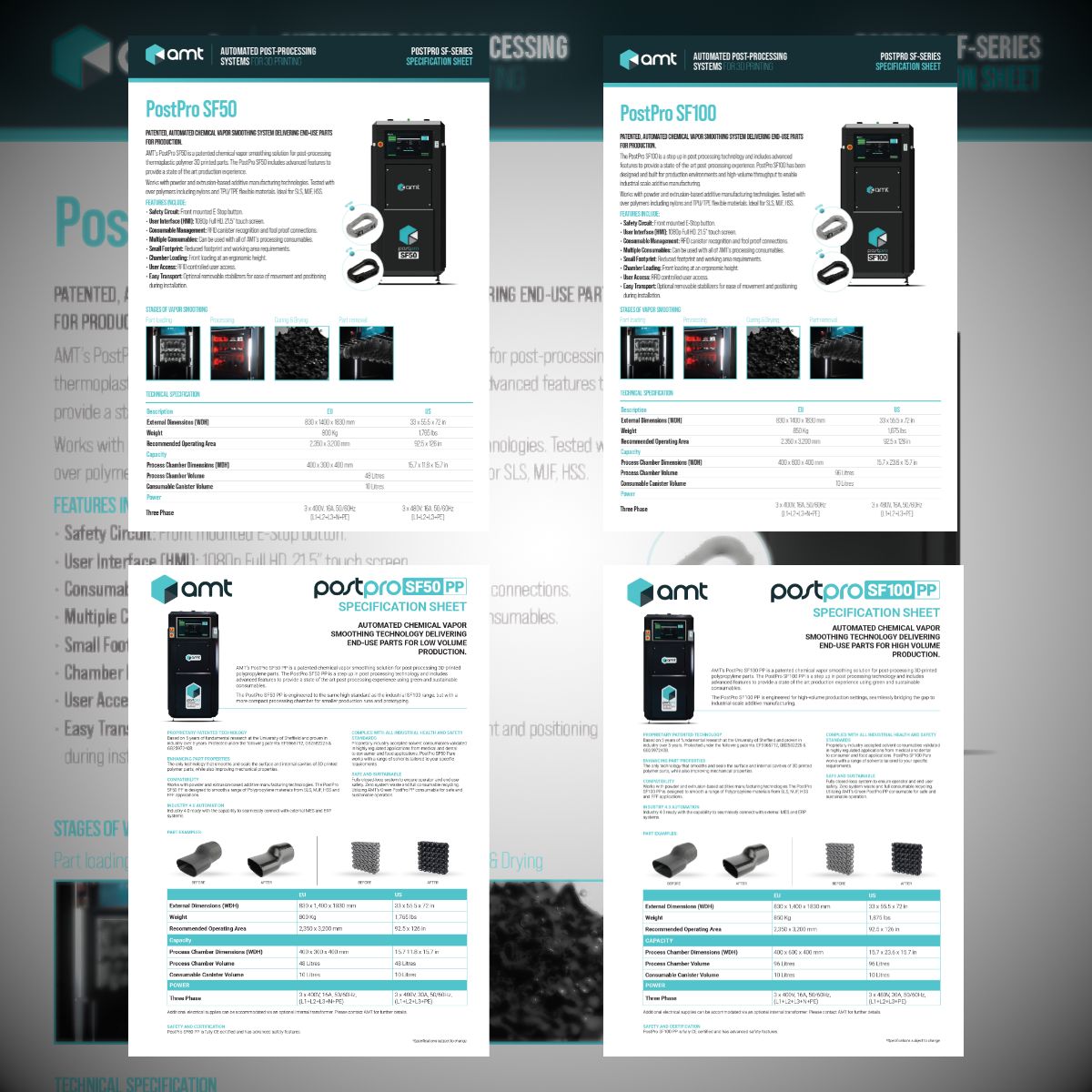
PostPro SF-Series

The PostPro SF-Series (SF50 Pure, SF100 Pure, SF50 PP, and SF100 PP) offers a comprehensive vapor smoothing solution for different healthcare applications.
-
Automated Chemical Vapor Smoothing Nachbearbeitungstechnologie
-
400 x 300 x 400 mm / 400 x 600 x 400 mm Chamber Dimension
-
48 Liter / 96 Liter Chamber Volume
Consistent and Functional
For dental and medical applications, the PostPro SF-Series (SF50 Pure, SF100 Pure, SF50 PP, and SF100 PP) provides outstanding surface enhancement. In order to minimize bacterial adherence and maintain cleanliness in medical devices, implants, and dental prosthesis, these technologies create smooth, sealed, and sterilizable surfaces on 3D printed parts.
In addition to greatly enhancing mechanical qualities like tensile strength and elongation at break, the vapor smoothing technique also makes treated parts far more functional and long-lasting for use in medicine. This is especially helpful for dental prosthesis, load-bearing implants, and surgical tools that need to be both aesthetically pleasing and structurally sound.
These technologies are perfect for sophisticated medical components because they can produce a consistent, high-quality finish across various geometries that would be challenging to finish using traditional methods.
Industry 4.0 Adherent
The PostPro SF-Series is perfect for contemporary medical manufacturing settings because of its sophisticated automation and Industry 4.0 adherence. They can accommodate several production scales, from small-batch specialized medical devices to larger-scale dental appliance production, thanks to processing chambers that range in capacity from 50 to 100 liters.
The systems may easily interface with external Enterprise Resource Planning (ERP) and Manufacturing Execution Systems (MES) software, guaranteeing effective workflow integration and enabling real-time production monitoring and quality control. This degree of automation lowers manual labor, enhances part consistency, and aids in upholding the stringent quality requirements necessary for the production of medical devices.
For medical applications, the capacity to monitor and trace every component through the completion process is very beneficial for quality control and regulatory compliance.
Broad Compatibility
SLS, MJF, HSS, SAF, FFF, and FDM with their own unique 3D printing materials are just a few of the many 3D printing technologies that the PostPro SF-Series is compatible with. Because of its adaptability, medical and dental practitioners can use a single system to convert surgical guides and dental models into personalized implants and prosthesis made of various materials.
The technology offers a complete solution for a range of medical manufacturing needs and works especially well with materials like nylon and polypropylene that are used in medical applications. This wide range of compatibility guarantees that medical device manufacturers and healthcare facilities can take advantage of the most recent developments in 3D printing materials without sacrificing the quality of the surface finish.
It also provides flexibility for future material developments, making the PostPro SF-Series a future-proof investment for evolving medical manufacturing requirements.
Safe and Validated
In healthcare applications, safety and regulatory compliance are crucial, and the PostPro SF-Series is excellent in these areas. For highly regulated applications, such as medical, dental, and food-safe products, these systems employ patented, industry-accepted solvent consumables that have undergone comprehensive validation.
By reducing exposure to chemicals and vapors during the finishing process, the fully closed-loop system guarantees the safety of operators and end users. Furthermore, the features of complete consumable recycling and zero system waste are consistent with sustainable manufacturing methods.
For medical and dental professionals working under stringent regulatory criteria, such as FDA guidelines or ISO 13485 standards for medical devices, the PostPro SF-Series is especially appealing due to its safety, compliance, and sustainability intertwined with material traceability and process documentation.
Applications
Orthodontics and Dental Prosthetics
Seal and smoothen 3D printed dental models, crowns, bridges, dentures, and orthodontic appliances to enhance the surface finish, make them more comfortable and resistant to bacterial growth.
Custom Medical Devices and Implants
Vapor smoothen custom implants, prosthetics, and medical devices to improve mechanical properties and surface quality to make them durable and suitable for medical use.
Surgical Guides and Tools
Smoothen, enhance and seal surfaces of 3D printed surgical guides and tools to make them easily sterilizable to maintain hygiene standards in medical settings.
Medical Research and Prototyping
Post-process 3D printed prototypes and research models to ensure repeatable results, which is essential for reproducible research outcomes.
Contact our team of professionals!
We're Here to Support Your Healthcare Journey